Seal Inspection Technologies for Solid Dose Pharmaceutical Packaging
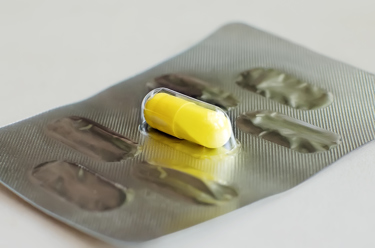
Blister packaging is one of the most practical and convenient packaging formats for tablets and capsules. Blister packaging is specifically used for tablets and capsules to pack them in a convenient and controlled way for easy patient use.
Blister packaging is designed for long and tamper-proof shelf life by eliminating moisture ingress and contamination. In today’s drug delivery systems where certain drugs are extremely sensitive to moisture and Oxygen, it is critical to ensure the package integrity of blister packs to ensure that product quality is not compromised.
Vacuum decay is a non-destructive container closure integrity testing method for blister packaging. PTI offers two technologies for integrity testing of multi-cavity blister packaging.
The OptiPac One-Touch Tool-less Leak Detection System is a deterministic non-destructive technology designed specifically for multi-cavity blister packs. OptiPac utilizes volumetric imaging under vacuum with topographic imaging to detect the presence and location of leaks. The OptiPac is engineered and designed with One-Touch Technology to achieve a rapid test cycle requiring no changeover or sample preparation. Operators simply place the blister package on the test plate and press the START button. Blister packs can also be placed in any orientation or position. Within seconds, the operator sees a definitive pass/fail result, and a visual identification of blister cavity defects. OptiPac technology is unique in that it can provide rapid detection of sub-5-micron defects depending on blister cavity volume. OptiPac requires no tools for different blister formats.
